Alzheimer's Clinical Study
MEMORIES ARE WHAT MAKES US HUMAN
We are seeking individuals between the ages of 55-80 who have been diagnosed with Alzheimer’s Disease to participate in a clinical research study.

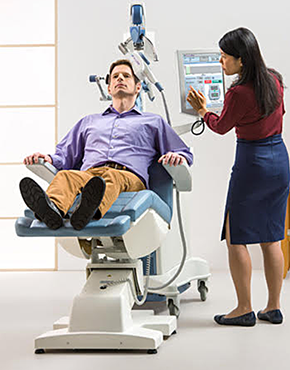
Transcranial magnetic stimulation, often referred to as TMS is a noninvasive procedure that uses magnetic fields to stimulate nerve cells in the brain to improve symptoms of depression. TMS is typically used when antidepressant medications haven’t been effective, have ceased working, or as an alternative to medication.
TMS involves delivering magnetic pulses to specific parts of the brain.
While there may be some minor discomfort at the treatment site (where the device touches your head), it generally subsides within the first week or treatment. There is no sedation, or impact on your alertness. You can read, watch TV, or talk with your treatment coordinator during your session, and you can drive home immediately after treatment.
A typical initial course of treatment is about 19-37 minutes daily over 4-6 weeks.
A vast majority of commercial and Medicare plans have recognized the effectiveness of treating depression with TMS Therapy and now cover TMS as part of their plans.
TMS does not circulate in the blood throughout the body, so it does not have side effects like weight gain, sexual dysfunction, nausea, dry mouth, sedation, etc. The most common side effects reported during clinical trials were headache and scalp discomfort — generally mild to moderate — occurring less frequently after the first week of treatment.
No. TMS Therapy involves a unique method of using pulsed magnetic fields for a therapeutic benefit. The intensity of the magnetic field is similar to that of an MRI. These techniques differ radically from the popular use of low intensity, static magnetic fields. Those products deliver weak and undirected static fields that are not capable of activating brain cells. The activation and stimulation of brain cells is a key part of why TMS is so effective.
1. Carpenter LL, et al. (2012). Transcranial Magnetic Stimulation (TMS) for Major Depression: A Multisite, Naturalistic, Observational Study of Acute Treatment Outcomes in Clinical Practice. Depres- sion and Anxiety, 29(7):587-596. www.ncbi.nlm.nih.gov/pubmed/22689344
2. George MS, et al. (2010). Daily Left Prefrontal Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder: A Sham-Controlled Randomized Trial. Arch Gen Psychiatry, 67(5):507-516. www.ncbi.nlm.nih.gov/pubmed/20439832
3. Dunner DL, et al. (2014). A Multisite, Naturalistic, Observational Study of Transcranial Mag- netic Stimulation (TMS) for Patients with Pharmacoresistant Major Depressive Disorder: Durability of Benefit Over a 1-Year Follow-Up Period. J Clin Psychiatry. 75(12):1394-1401. www.ncbi.nlm.nih.gov/ pubmed/25271871
4. O’Reardon JP, et al. (2007). Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biol Psychiatry, 62(11):1208-1216. www.ncbi.nlm.nih.gov/pubmed/17573044
Stedman Clinical Trials offers professional recruitment, trial and administrative services across a variety of indications, with a central focus on psychological disorders and neuro-degenerative diseases such as Alzheimer’s. Choosing to participate in a study is an important personal decision. Talk with your doctor and family members or friends about deciding to join a research study.

MEMORIES ARE WHAT MAKES US HUMAN
We are seeking individuals between the ages of 55-80 who have been diagnosed with Alzheimer’s Disease to participate in a clinical research study.

YOU DON'T HAVE TO LIVE WITH LONELINESS
We are seeking individuals ages 18 and older who have been diagnosed with major depression, and are currently being treated with an antidepressant, to participate in a clinical research study.